Two kids come home from school with red, crusty sores around their noses. A parent panics - is this just a scratch or something worse? Meanwhile, an older man notices his lower leg swelling, turning bright red, and burning with heat. He thinks it’s a bruise. Both situations are skin infections, but they’re not the same. One is impetigo - common, contagious, and usually mild. The other is cellulitis - deeper, dangerous, and can turn life-threatening fast. Knowing the difference isn’t just about labels; it’s about picking the right treatment before things get worse.
What Impetigo Looks Like - And Why It Spreads So Fast
Impetigo is the classic "school sores" infection. It hits kids hardest, especially between ages 2 and 5. You’ll see it around the nose, mouth, or on exposed skin after a bug bite or scrape. The two types are easy to tell apart if you know what to look for.
Nonbullous impetigo (70% of cases) starts as small red blisters that burst quickly. Within hours, they leave behind sticky, golden-yellow crusts - like honey dried on the skin. It doesn’t hurt much, but it itches. The bacteria behind this? Usually Staphylococcus aureus, sometimes Streptococcus pyogenes. It doesn’t need broken skin to invade - it can slip right through healthy skin if the immune system is down.
Bullous impetigo is less common. It forms bigger, fluid-filled blisters (2-5 cm wide) that look like tiny water balloons. They’re thin, clear, and yellowish. When they pop, they leave a red ring around the area. This type is almost always caused by Staphylococcus aureus making a toxin that splits the skin layers.
Here’s the kicker: impetigo is contagious as hell. It spreads through touch - sharing towels, toys, or even just hugging. Kids in daycare or classrooms are ground zero. A child with impetigo should stay home until they’ve been on antibiotics for at least 24 hours. Otherwise, half the class could end up with crusty sores by Friday.
Cellulitis: When the Infection Goes Deep
Cellulitis isn’t just a rash. It’s an infection that burrows into the deeper layers of skin - the dermis and fat underneath. It doesn’t form blisters or crusts. Instead, it swells, heats up, and turns angry red. The edges are blurry, not sharp. It often starts where the skin was broken - a cut, a fungal crack between the toes, a diabetic ulcer, or even a spider bite.
Unlike impetigo, cellulitis doesn’t just sit on the surface. It can spread fast. If left untreated, it can enter the bloodstream and cause sepsis. That’s why it’s one of the top 30 reasons people end up in the hospital. The CDC says skin infections like this make up 14.5 million outpatient visits a year in the U.S. alone.
Most cases are caused by Streptococcus bacteria - especially group A. But Staphylococcus aureus is also a big player. And if you’ve had cellulitis before, or you’re diabetic, have poor circulation, or take steroids, your risk shoots up. Elderly people are especially vulnerable.
One thing that trips people up: erysipelas. It looks like cellulitis - bright red, hot, swollen - but the borders are sharp, like a line drawn with a ruler. That’s usually Streptococcus doing its thing. Doctors sometimes treat it like a more severe version of cellulitis, but the treatment is similar.
Antibiotic Choices: What Works, What Doesn’t
Both infections are bacterial. Both need antibiotics. But you don’t treat them the same way. Using the wrong drug wastes time, fuels resistance, and risks complications.
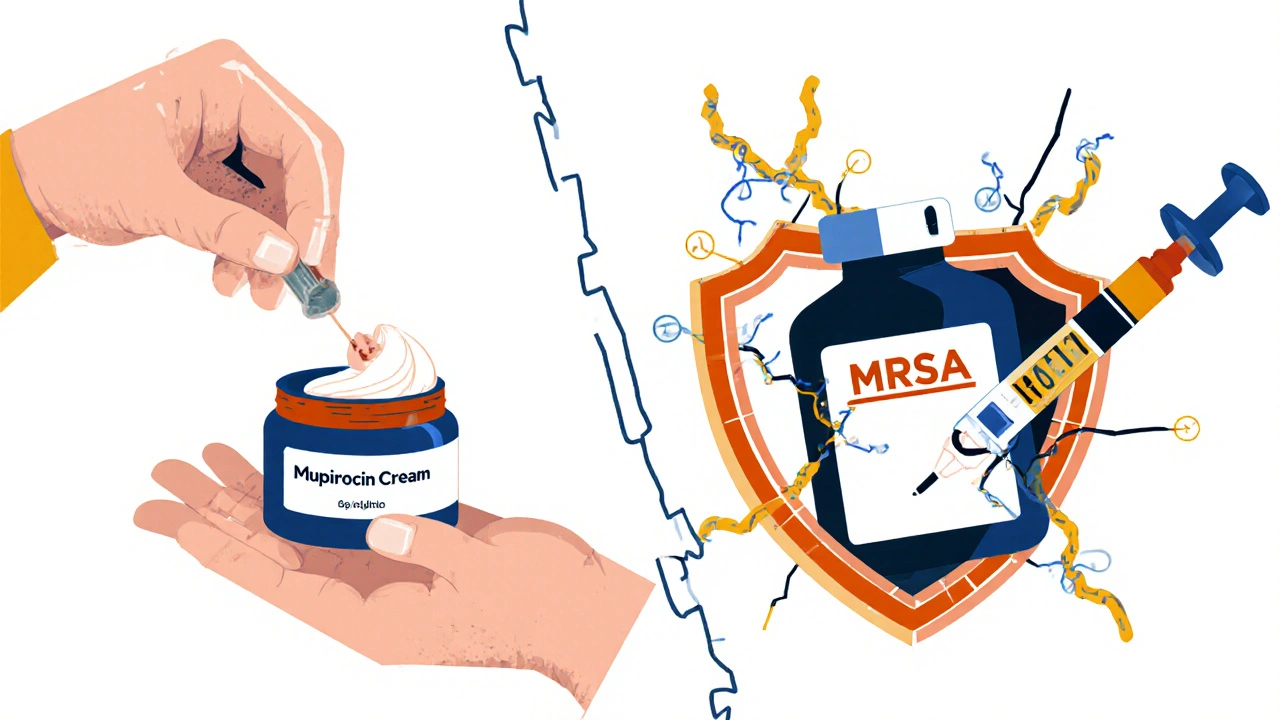
For impetigo, if it’s small and localized - say, two or three spots - topical mupirocin cream (Bactroban) works great. Studies show it clears up 90% of cases in under a week. Just apply it three times a day for 5-10 days. Wash the area first with soap and water, pat dry, then dab it on.
If the infection is widespread - covering the face, arms, legs - you need oral antibiotics. In the UK, flucloxacillin is the go-to. It’s a penicillin-based drug that kills staph and strep. In France, they use amoxicillin-clavulanate more often. Belgium? Mostly flucloxacillin too. The key is matching the drug to the most likely bug. Flucloxacillin covers staph well. If you’re allergic to penicillin, alternatives like clarithromycin or clindamycin work.
For cellulitis, you need something that gets deep into tissue. Oral antibiotics are standard unless the patient is very sick, has a fever, or can’t swallow pills. Then they go to the hospital for IV antibiotics.
Again, flucloxacillin is first-line in the UK. In the U.S., doctors often pick cephalexin or dicloxacillin - similar drugs. In France, amoxicillin is now the top choice for cellulitis, even though it doesn’t cover staph as well. Why? Because strep is the main culprit, and they’re trying to avoid overusing drugs that target staph.
Here’s the problem: MRSA. Methicillin-resistant Staphylococcus aureus doesn’t respond to flucloxacillin, cephalexin, or any penicillin-type drug. If someone has a history of MRSA, lives in a crowded area, or their infection isn’t improving after 48 hours, doctors suspect MRSA. Then they switch to clindamycin, doxycycline, or trimethoprim-sulfamethoxazole. In serious cases, vancomycin or linezolid might be needed - but only in hospital.
Why Regional Differences Matter
Why does France use amoxicillin for cellulitis while the UK sticks with flucloxacillin? It’s not random. It’s about local resistance patterns.
Over the last decade, MRSA rates in UK hospitals have dropped thanks to strict hygiene rules. So flucloxacillin still works well. In France, strep is the bigger concern, and they’ve shifted toward amoxicillin to reduce pressure on staph-targeting drugs. Belgium has no national guidelines - so doctors guess, which leads to inconsistency.
What does this mean for you? If you’re in the UK and your doctor prescribes flucloxacillin for impetigo or cellulitis, that’s normal. If you’re in the U.S., you’re more likely to get cephalexin. In the UK, if your child’s sores aren’t clearing up after 5 days on mupirocin, it’s time to see a doctor - you might need oral antibiotics.
Don’t assume what works in one country works everywhere. Resistance is local. Your doctor should know what’s common in your area.
What Happens If You Delay Treatment
Impetigo might seem harmless - it’s just crusts, right? But if you wait too long, it can spread to other parts of the body or to family members. In rare cases, strep-related impetigo can trigger post-streptococcal glomerulonephritis - a kidney problem that shows up weeks later.
Cellulitis? Delaying treatment is risky. Every hour counts. Studies show that starting antibiotics within 48-72 hours cuts the chance of hospitalization by half. If you wait five days, the infection can spread to muscles, bones, or the blood. Sepsis doesn’t come with a warning sign. It just shows up - fever, chills, confusion, rapid heartbeat. By then, it’s too late for a simple pill.
Parents often delay because they think it’s "just a rash." Adults ignore it because they’re busy. But if your skin is hot, swollen, and red - especially if it’s spreading - don’t wait. Go to the clinic. Get a diagnosis. Start antibiotics.
Prevention: Simple Steps That Actually Work
You can’t always stop an infection, but you can make it much less likely.
- Wash cuts and scrapes right away with soap and water. Dry them well.
- Don’t pick at scabs or crusts. That’s how you spread it to other parts of your body.
- Never share towels, razors, or clothing with someone who has impetigo or cellulitis.
- If you have eczema, keep your skin moisturized. Dry, cracked skin is an open door for bacteria.
- For diabetics: check your feet daily. A small sore can turn into cellulitis before you notice.
- Teach kids to cover their sores with a bandage and wash hands often.
Hygiene isn’t just about being clean - it’s about breaking the chain of transmission. In households where one person has impetigo, others are 5 times more likely to catch it. But if you wash hands after touching the area and change bedding daily, you can stop the spread.
When to See a Doctor - And What to Ask
You don’t need to panic over every red spot. But here’s when to call:
- Sores that spread quickly or look like blisters
- Redness that’s warm, swollen, and expanding
- Fever or chills along with skin changes
- Diabetes, poor circulation, or a weak immune system + any skin break
- No improvement after 2-3 days of home care
When you see the doctor, ask:
- "Is this impetigo or cellulitis?"
- "Could this be MRSA?"
- "Do we need a swab or culture?"
- "Is this antibiotic the right one for our area?"
- "What signs should I watch for that mean it’s getting worse?"
Doctors aren’t mind readers. If you don’t mention that your child went to school yesterday with sores, or that your leg swelled up after a mosquito bite, they might miss the connection.
Can impetigo turn into cellulitis?
Not directly. Impetigo stays on the surface; cellulitis digs deeper. But if you scratch impetigo sores and introduce bacteria into a deeper wound - like a cut or eczema crack - you can trigger cellulitis. That’s why it’s important to keep sores covered and avoid scratching.
Are topical antibiotics enough for impetigo?
Yes - if the infection is limited to one or two small areas. Mupirocin cream works well for that. But if the sores are widespread, cover your face, or keep coming back, you’ll need oral antibiotics. Topical treatment alone won’t stop it from spreading to others.
How long does it take for antibiotics to work on cellulitis?
You should start seeing improvement within 48 hours - less redness, less swelling, less pain. If it’s getting worse after two days, or you develop a fever, go back to the doctor. You might need a stronger antibiotic or IV treatment.
Can you get cellulitis without a cut?
Yes. Bacteria can enter through tiny breaks you don’t even notice - like dry, cracked skin from eczema, athlete’s foot, or even a small insect bite. People with poor circulation or diabetes are especially at risk because their skin heals slower and immune response is weaker.
Is MRSA common in skin infections?
It’s not rare. In hospitals, MRSA is a big problem. In the community, it’s growing - especially in places with close contact like gyms, prisons, or childcare centers. About 1 in 10 skin infections in some areas are MRSA. If your infection doesn’t respond to standard antibiotics, your doctor should test for it.
Do I need to finish the whole course of antibiotics?
Yes - even if you feel better. Stopping early leaves behind the toughest bacteria. They multiply and become resistant. That’s how superbugs like MRSA spread. Finish every pill, even if the rash is gone.
What Comes Next?
Antibiotic resistance is getting worse. That’s why doctors are starting to use cultures and sensitivity tests more often - especially for recurrent infections. In five years, we’ll likely see faster tests that tell you in hours whether you have MRSA or a regular staph infection - and which antibiotic will actually work.
For now, the best defense is awareness. Know the difference between a crusty sore and a hot, swollen leg. Don’t ignore skin changes. Treat small wounds fast. Ask your doctor about local resistance patterns. And never stop antibiotics early.
These infections are common. But they’re not harmless. With the right knowledge, you can stop them before they stop you.
