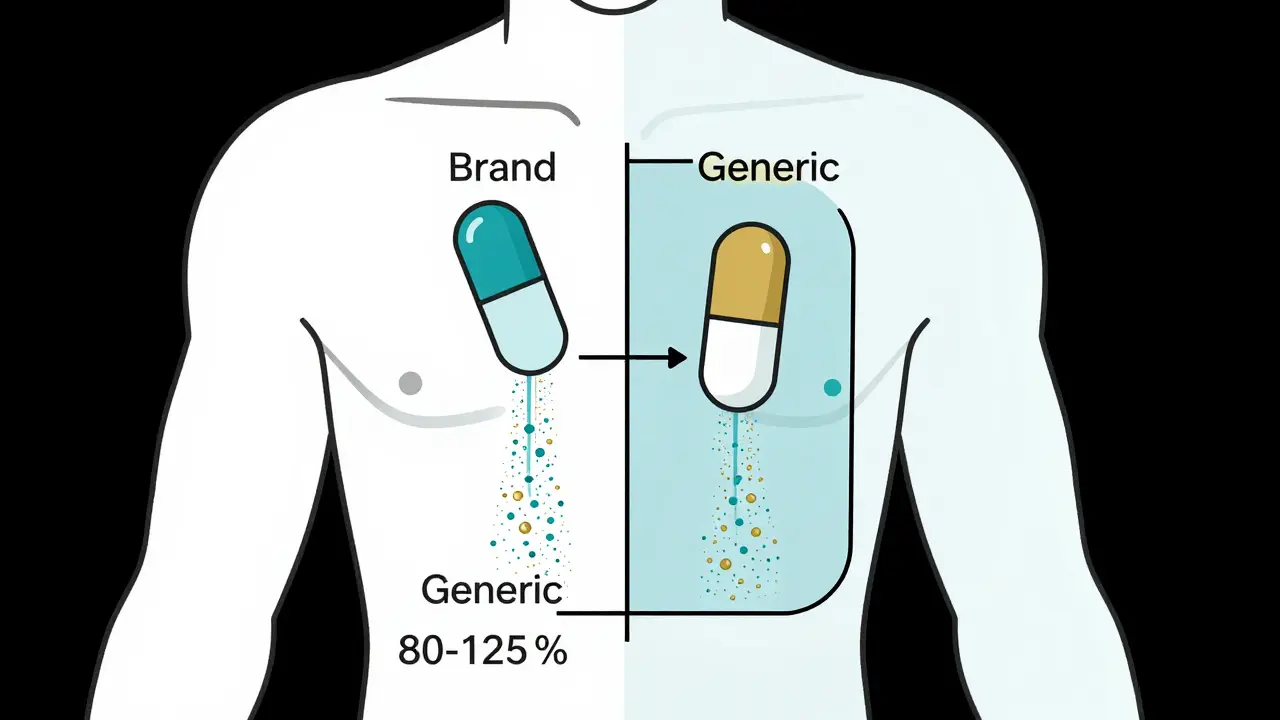
When you pick up a generic prescription, you might wonder: is this really the same as the brand-name drug you’ve been taking? You’ve probably heard the number 80-125% thrown around - and it’s easy to assume it means generic drugs can have up to 25% less or 25% more of the active ingredient. That’s not just wrong. It’s dangerously misleading.
What the 80-125% Rule Actually Measures
The 80-125% range isn’t about how much active ingredient is in the pill. It’s about how much of that ingredient actually gets into your bloodstream - and how fast. This is called bioavailability. The rule is a statistical tool used by the FDA and other global regulators to prove that a generic drug behaves the same way in your body as the brand-name version.
Here’s how it works: In a bioequivalence study, healthy volunteers take both the brand-name drug and the generic version, usually in a random order, with a washout period in between. Blood samples are taken over 24 to 72 hours to measure two key things: the total amount of drug absorbed (AUC), and how quickly it peaks in your blood (Cmax). These numbers are then compared.
The FDA doesn’t just look at the average difference. They calculate a 90% confidence interval - a range that shows where the true difference between the two drugs likely falls. For the generic to be approved, the entire 90% confidence interval must fall between 80% and 125% of the brand drug’s values. That’s the rule.
But here’s the twist: because these measurements are log-transformed for statistical accuracy, the 80-125% range isn’t symmetrical around 100%. It’s mathematically designed to reflect a ±20% variation on a logarithmic scale. That means the real-world average difference between generics and brand drugs is usually under 5%. In fact, the FDA analyzed over 2,000 studies between 2008 and 2012 and found that 98% of approved generics had point estimates within 95-105% of the brand drug.
Why 80-125% and Not 90-110%?
You might think: why not just require a tighter range, like 90-110%? That seems safer. But biology isn’t that neat.
People absorb drugs differently. Your age, what you ate before taking the pill, your liver enzymes, even your gut bacteria - all of these affect how much drug enters your bloodstream. A 10% variation in absorption is normal between two doses of the exact same brand drug taken by the same person on different days.
The 80-125% range accounts for this natural variability. If regulators used a fixed ±10% rule, they’d reject perfectly safe and effective generics just because of normal biological noise. The 90% confidence interval requirement makes the standard even stricter. For a generic to consistently pass, its true bioavailability must be within 5-10% of the brand drug. That’s not a loophole - it’s a safeguard.
What About High-Risk Drugs Like Warfarin or Levothyroxine?
Not all drugs are created equal. Some have a narrow therapeutic index - meaning the difference between an effective dose and a toxic one is tiny. For drugs like warfarin (a blood thinner), levothyroxine (for thyroid function), or phenytoin (for seizures), even a 10% difference in absorption can be dangerous.
That’s why the FDA uses tighter standards for these. For certain narrow therapeutic index drugs (NTIDs), the acceptable range is 90-111%. The 80-125% rule doesn’t apply here. The FDA tracks these drugs in a special database and requires more complex testing, including multiple-dose studies and sometimes clinical endpoint data - not just blood levels.
So if you’re on warfarin and your pharmacist switches you to a generic, it’s not because the rule is looser. It’s because the generic was held to a higher standard.
Real-World Evidence: Do Generics Actually Work?
Myths about generics persist - especially online. On Reddit and pharmacy forums, people still claim generics are “weaker” or “fillers.” But the data doesn’t back that up.
A 2016 study in JAMA Internal Medicine followed over 2 million patients on cardiovascular drugs. Some stayed on brand-name medications. Others switched to generics. The results? No difference in heart attacks, strokes, or hospitalizations. The same pattern shows up for antidepressants, seizure meds, and blood pressure drugs.
The FDA’s Sentinel Initiative, which monitors 200 million patient records, found no increase in adverse events for 94% of generic drugs between 2015 and 2020. In fact, generic drugs have been used safely for over 30 years. The Supreme Court upheld the FDA’s bioequivalence standards in 2022, noting they’ve been proven by decades of real-world use.
Even doctors who were once skeptical have changed their minds. Dr. Jerry Avorn from Harvard Medical School pointed out that the average difference between brand and generic drugs is just 3.5% - less than the natural day-to-day variation in your own body’s absorption.
Why Do Pharmacists Keep Explaining This?
Because patients still worry. A 2020 survey of 1,200 U.S. pharmacists found that 78% explain the bioequivalence rule to patients at least once a week. Many patients fear generics are “cheap” or “inferior.”
But here’s the truth: generic manufacturers don’t cut corners. They have to prove their drug matches the brand - down to the last detail. The active ingredient must be identical. The inactive ingredients (fillers, dyes, binders) must be safe and not interfere with absorption. The manufacturing process must be validated. And every batch is tested.
When a pharmacist says, “This generic is just as good,” they’re not being reassuring. They’re citing federal law backed by decades of science.
What Happens When a Generic Fails?
Not every generic gets approved. About 32% of initial applications to the FDA are incomplete or fail bioequivalence testing. That’s not because companies are lazy - it’s because getting it right is hard.
Some drugs are tricky. Think of complex formulations: extended-release pills, inhalers, topical creams, or injectables. For these, measuring blood levels isn’t enough. The FDA now requires more advanced testing - sometimes even clinical trials - to prove effectiveness.
For example, a generic asthma inhaler might have the same active ingredient, but if the particle size or spray pattern is off, the drug won’t reach the lungs properly. That’s why the FDA has issued specific guidance for over 1,600 complex products.
When a generic fails, the company goes back to the lab. They tweak the formulation. They run new tests. And they try again. The system is designed to reject anything that doesn’t meet the standard - not to let anything slide.
Global Standards: Is This Rule Used Everywhere?
The 80-125% rule isn’t just an American thing. The European Medicines Agency (EMA), Health Canada, Australia’s TGA, and over 50 other countries use the same standard. It’s been harmonized by the International Council for Harmonisation (ICH).
Some countries, like Brazil and India, have modified rules for certain drugs, especially where access is a bigger concern than precision. But for most high-income nations, the 80-125% rule with a 90% confidence interval is the gold standard.
That’s why a generic made in India and approved by the FDA will behave the same as one made in Germany - if it passes the test.
Why This Matters for You
Generics save the U.S. healthcare system over $370 billion a year. They’re not just cheaper - they’re accessible. Without them, millions of people couldn’t afford their medications.
But if you’re worried your generic isn’t working, here’s what to do:
- Don’t assume it’s the drug. Changes in how you feel could be from stress, diet, sleep, or another condition.
- If you notice a real change - like new side effects or reduced effectiveness - talk to your doctor. Don’t switch back to brand without a reason.
- Ask your pharmacist: “Is this generic FDA-approved for bioequivalence?” If they hesitate, they’re not following best practice.
- For high-risk drugs like warfarin or levothyroxine, your doctor may choose to keep you on one brand for consistency - but that’s a clinical decision, not a regulatory one.
The bottom line? The 80-125% rule isn’t a loophole. It’s a rigorous, science-backed standard that ensures your generic drug works just like the brand - and has for over 30 years.
Does the 80-125% rule mean generics can have 25% less active ingredient?
No. The 80-125% range refers to the total amount of drug absorbed into your bloodstream (bioavailability), not the amount of active ingredient in the pill. All generics must contain the exact same active ingredient as the brand-name drug. The rule ensures that how your body processes the drug is nearly identical - not that the pill contains less medicine.
Are generic drugs less effective than brand-name drugs?
No. Large-scale studies involving millions of patients show no meaningful difference in effectiveness between brand-name and FDA-approved generic drugs. For example, a 2016 JAMA study found identical outcomes for heart medications whether patients took the brand or generic version. The FDA’s own analysis shows 98% of generics fall within 95-105% of the brand’s absorption rate.
Why do some people say generics don’t work for them?
Sometimes, it’s not the drug - it’s the change. Switching from one pill to another - even if it’s bioequivalent - can trigger placebo effects or make people more aware of side effects they didn’t notice before. Inactive ingredients (like fillers or dyes) can also cause minor reactions in sensitive individuals. But if you’re having real problems - like worsening symptoms or new side effects - talk to your doctor. They can check your blood levels or switch you to a different generic or brand.
Are all generic drugs tested the same way?
Most are - but not all. Standard immediate-release pills follow the 80-125% bioequivalence rule using blood tests. But complex drugs like inhalers, topical creams, or extended-release capsules require more advanced testing. The FDA has special guidelines for over 1,600 complex products, sometimes requiring clinical studies instead of just blood measurements. If a drug is hard to test, the FDA demands more proof.
Can I trust generics from other countries?
Only if they’re approved by your country’s regulator - like the FDA in the U.S. or the EMA in Europe. Many generics made overseas meet the same standards as U.S.-made ones. But buying unapproved generics online is risky. They may be counterfeit, improperly stored, or lack proper testing. Always get your generics from a licensed pharmacy - whether they’re made locally or imported.